The request to consolidate lawsuits against pharmaceutical companies Novo Nordisk and Eli Lilly, who are under fire over the dangerous side effects linked to their explosively popular diabetes and weight loss drugs, was granted in February by the U.S. Judicial Panel on Multidistrict Litigation (JPML). The panel ruled that the drugs are similar enough that consolidating the cases would streamline pretrial discovery and other proceedings. All current and future case rulings are now in the hands of U.S. District Judge Gene Pratter in the Eastern District of Pennsylvania.
The diabetes weight loss drugs lawsuits allege that drugmakers failed to warn patients and their physicians about the risks of gastroparesis (stomach paralysis), an extremely dangerous condition that prevents proper digestion and can lead to dehydration, malnutrition, and buildup of undigested food in the stomach.
In January, the plaintiffs’ lawyers requested to centralize the cases into multidistrict litigation (MDL) in Louisiana, while the primary defendant, Nordisk, wanted them moved to North Carolina. Pennsylvania was chosen for its proximity to Nordisk’s U.S. offices in New Jersey.
Nordisk said in a statement that it supports the MDL but believes the claims are false and will vigorously defend against them. Lilly, named in a much smaller number of lawsuits, objected to being included in the MDL.
Now that the diabetes weight loss drugs lawsuits have been transferred to MDL, lawyers expect thousands more lawsuits will be filed in the coming months.
Why Are Patients Filing Diabetes Weight Loss Drugs Lawsuits?
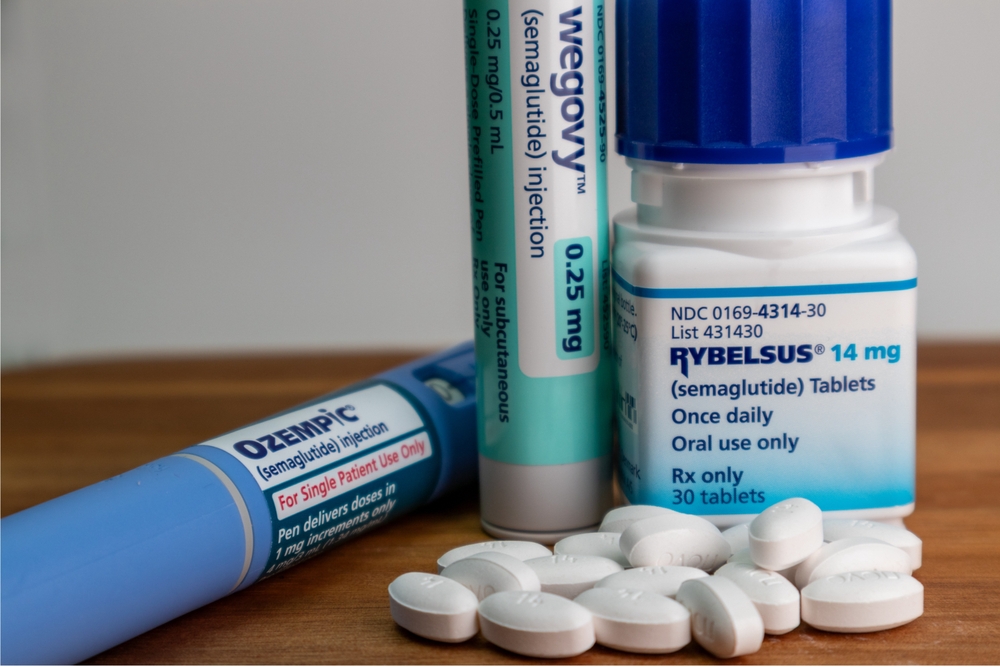
Ozempic, Mounjaro and similar medications are used to treat type 2 diabetes but have recently skyrocketed in popularity after celebrities and influencers began touting them as wonder drugs for weight loss. J.P. Morgan Research forecast that 30 million people will use them by 2030.
The drugs are classified as GLP-1 receptor agonists, which make the user feel fuller sooner while eating. Novo Nordisk's Ozempic, Wegovy, and Rybelsus contain semaglutide as their main ingredient, while Eli Lilly's Mounjaro features tirzepatide and Trulicity utilizes dulaglutide. These primary ingredients reduce food intake and increase insulin sensitivity. For diabetes patients, they are used in combination with other treatments and lifestyle changes to lower blood sugar and assist in weight loss.
The only one of the diabetes drugs approved by the FDA for weight loss is Wegovy; doctors have increasingly prescribed the other drugs as off-label medications to patients wishing to use them for that purpose, resulting in a nationwide shortage. This makes them more difficult to find for diabetes patients, who need them to stay healthy.
Mild gastrointestinal side effects, such as nausea, diarrhea, and stomach pain, are normal when taking semaglutide and tirzepatide and are noted on the packaging. The lawsuits have been filed due to a lack of warning regarding gastroparesis and other severe complications, including thyroid tumors, pancreatitis, acute kidney injury, and gallbladder disease. And because patients must stay on weight loss drugs indefinitely to see results, the risk of long-term side effects is significant.
The first weight loss drug lawsuit was filed in August 2023 by a diabetic woman who was hospitalized for intense vomiting, stomach pain, and gastrointestinal burning after using Ozempic and Mounjaro. Dozens of others with similar results soon filed their own claims, leading to the MDL.
Multidistrict Litigation for Dangerous Drug Lawsuits
When multiple federal lawsuits are filed by many plaintiffs over the same issue, multidistrict litigation helps move them more quickly and economically through the legal process. Product liability cases involving dangerous mass-produced drugs are a natural fit for MDL, which saves both plaintiffs and defendants time and money. It’s much easier to gather discovery documents, schedule testimony from witnesses and experts, and reach settlement agreements if they are managed within one court. That court is chosen based on where most of the witnesses, actions, and discovery materials are located; any cases that go to trial are returned to their originating court.
However, the MDL judge will select a few individual cases for “bellwether” trials, the verdicts of which indicate to the parties roughly what arguments will be successful and to what award amount. Successful plaintiffs in these trials often lead defendants to settle any remaining claims out of court.
Patients deserve to know that a prescribed medication will not harm them. If drugmakers purposely withhold information that can jeopardize patient safety, a dangerous drug lawsuit can hold them accountable. The creation of the weight loss drug MDL indicates progress toward appropriate financial compensation for plaintiffs. Hiring a reputable weight loss drug lawyer experienced in product liability, who will guide you through the process step-by-step, is the best way to reap these benefits. Trustworthy personal injury firms will always evaluate the strength of your claim during a free consultation and ask for payment only if you win.