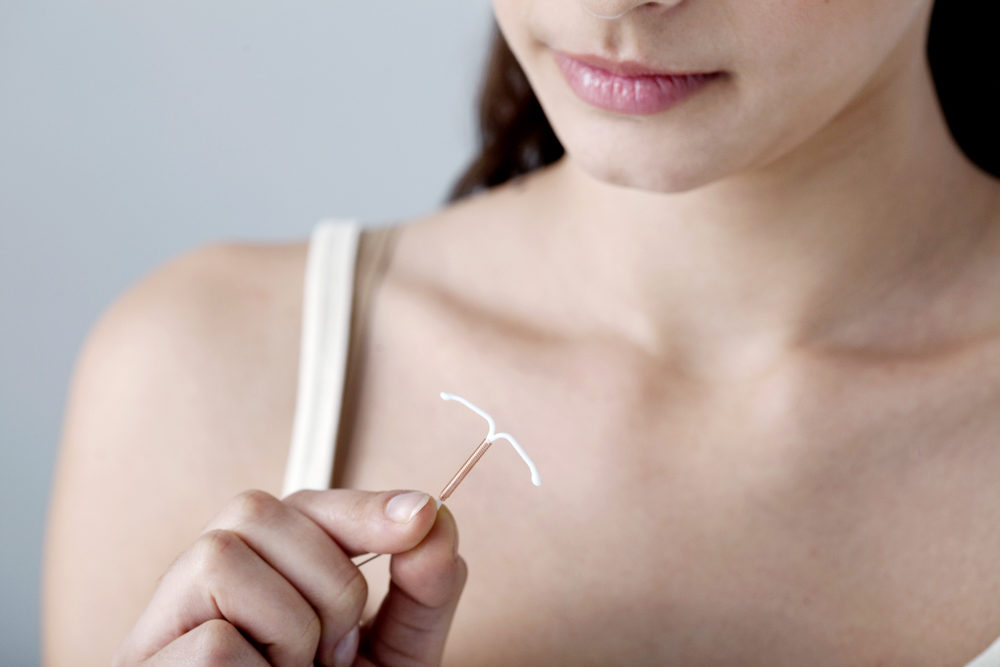
Choosing a birth control method can be overwhelming for women, with well over a dozen forms available to consumers. The Paragard IUD, introduced in 1988, has emerged as a popular choice in recent years. Given that some options require you to take pills daily or change out a ring monthly, intrauterine devices (IUDs) have become one of the most common contraceptive options in the U.S.
Intrauterine devices are inserted into the uterus, usually during a doctor’s visit. The process usually takes less than five minutes, and devices like Paragard are 99% effective and lasts up to 12 years.
But in rare cases, the Paragard can make life excruciatingly difficult for patients — namely by perforating or falling out of the uterus or breaking into pieces while inserted. While uncommon, issues with Paragard can cause severe pain, bleeding, and other health complications. The U.S. Food and Drug Administration (FDA) has received over 1,600 reports of Paragard breakage since 2010, and it’s almost certain that thousands more have gone unreported.
Over 100 Paragard lawsuits have been filed against the IUD's manufacturers, and they’ve been consolidated into multidistrict litigation (MDL) in the Northern District of Georgia. Multidistrict litigation dates back to the 1960s, and it is used when there are hundreds or thousands of cases across multiple courts that all involve the same problem. Ideally, MDL will save time and allow lawsuits to move through the legal system faster.
What is causing women to choose Paragard, and why are there so many problems being reported?
What Is Paragard?
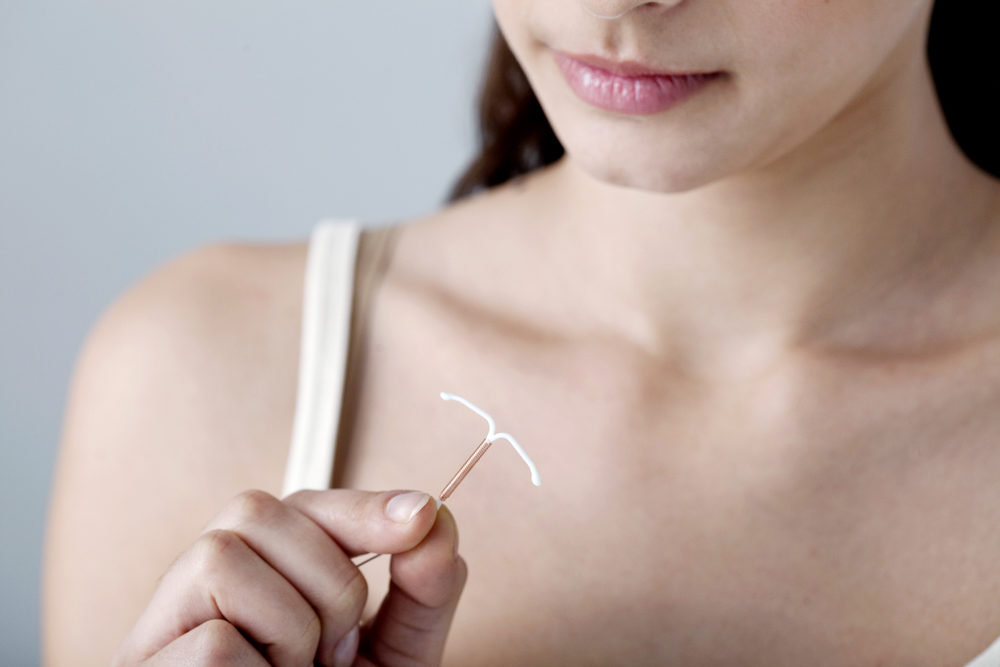
Paragard is the only copper IUD available in the United States. It has a T-shaped plastic frame and is wrapped in a thin layer of copper. The Paragard IUD has been marketed under various companies in the three decades it has been on the market, including GynoPharma, Duramed Pharmaceuticals, Teva Pharmaceuticals, and more. Teva sold Paragard to CooperSurgical in 2017 for $1.1 billion.
The basic idea of the IUD stems back to 1909 when a physician named Dr. Richard Richter inserted a ring made of silkworm gut into the uterus. He cut off the ends outside of the cervix, creating the first version of the string that hangs out to check and remove the device. The device went through many rounds of experimentation over the next few decades.
In 1969 there was the development of the plastic T-shape design and discovery of the efficacy of copper for pregnancy prevention. By the 1970s, 15 different companies were developing 17 different IUDs. The first three hit the market without incident; the fourth, the Dalkon Shield by the A.H. Robins Company, only spent three years on sale before the discovery that its unsealed removal string pushed bacteria into the uterus. This causes pelvic inflammatory disease, a painful condition that can destroy a woman’s ability to reproduce. A.H. Robins filed for bankruptcy after more than 400,000 lawsuits were filed against them. IUD usage dropped sharply until the Paragard appeared in 1988.
Paragard was developed during the 1970s by the Population Council and Finishing Enterprises, Inc. (FEI). It was not approved by the FDA until 1984, originally for just four years of continuous use. Improvements to the device prompted the FDA to extend that approval to six years, eight years and finally ten years.
Paragard is not the only IUD to cause suffering for thousands of women. The Mirena IUD, a hormonal device that lasts up to five years, has faced similar allegations. Its parent company, Bayer, settled 4,600 cases in 2018 for $12.2 million.
Benefits of Paragard
Hormonal birth control is known for side effects like nausea, headaches, and weight gain. Because Paragard and similar intrauterine devices are hormone-free and have fewer effects, they are often favored. The risk of pregnancy is also low, with less than 1% of women becoming pregnant in the first year of use. Birth control pills, on the other hand, fail about 7% of the time.
Paragard can last 10-12 years, and it doesn’t carry severe risks like blood clots because it doesn’t have hormones. Like most IUDs, Paragard is very small, measuring about 1” wide and 1” high. IUDs are inserted with a speculum and can be painful at first. For most women, the pain subsides, and there are few side effects after IUD insertion. And, of course, they are a “set-it-and-forget” method of contraception.
An IUD is much cheaper than birth control pills in the long run. While pills can cost up to $50 a month without insurance, an IUD without insurance has a one-time cost of anywhere from $500-1300. Even at the highest price, if used for the full ten years, an IUD’s monthly cost would only be about $11.
Complications Of Paragard
While Paragard is advertised as an effortless way to prevent pregnancy, complications can occur. If the device becomes dislodged, perforates the uterus, or breaks into pieces while inserted, the patient may need surgery, leading to medical bills, missed unemployment, and unexpected pain and suffering.
In a recent instance, a woman in North Carolina filed a suit in the U.S. District Court for the Eastern District of North Carolina. She alleges that her doctor followed Paragard’s instructions for removal, but only part of the IUD came out, and a piece was lodged in her uterus. As a result, she was left injured with a loss of reproductive health.
Another woman opted for the Paragard due to its lack of hormones. When she had it removed after five years, she immediately knew a piece of it had broken inside her. She underwent surgery to find and remove the missing piece. She said she had never been warned of the possibility of breakage.
According to voluntary FDA reports, in the cases filed since 2013, there have been 3,186 cases of breakage. Nearly 2,000 of those were considered serious, and more than 100 of them caused life-threatening injuries.
Along with the physical injury, if the Paragard is dislodged without your knowledge, it may not prevent pregnancy. While Paragard is popular among healthcare providers and patients alike, it’s essential to make yourself aware of the potential risks.
Despite its controversial past, Paragard has been recalled just once in 2014. Its manufacturer at the time, Teva Pharmaceuticals, recalled some of the devices for not meeting sterility standards. It has never been recalled for lawsuit-related issues like breakage problems.