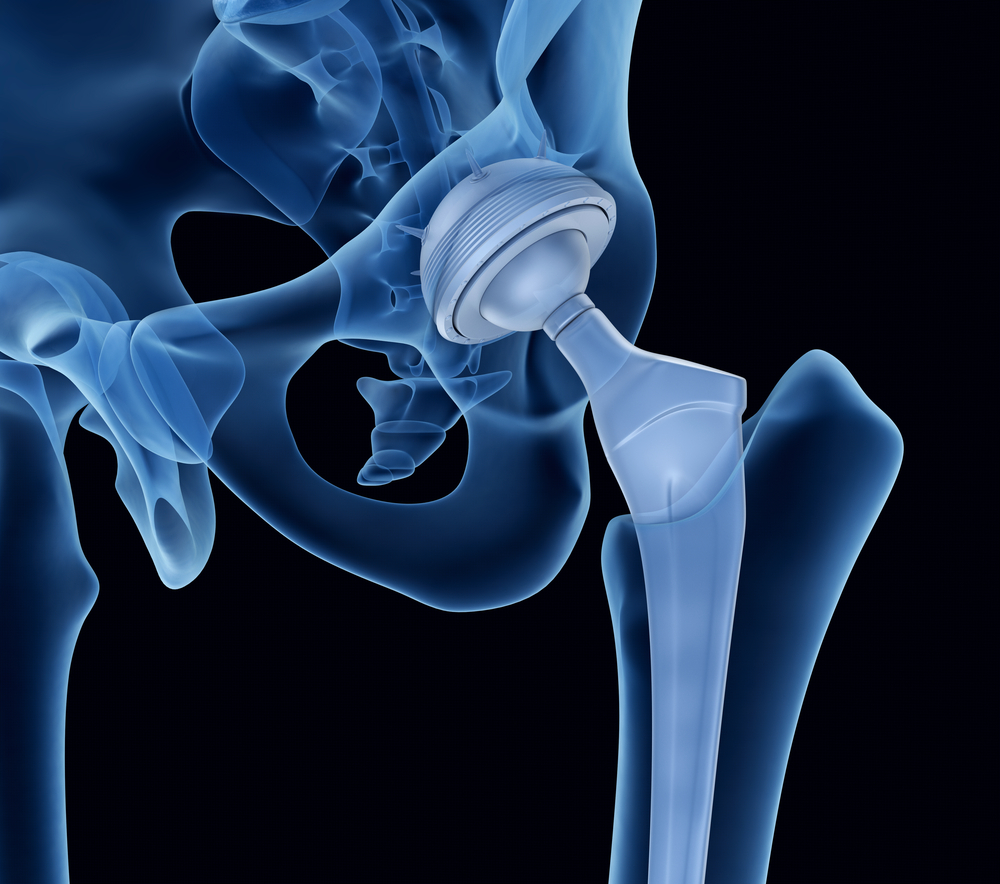
Patients who underwent total hip replacement surgeries using a medical device meant to revolutionize the treatment are suing its manufacturers after a design defect failed catastrophically.
A recent investigation by KFF Health and CBS News found that hundreds of Profemur brand artificial hip implants have broken apart at the dual modular “neck,” causing patients to suddenly collapse and lose their ability to walk or stand. The simplest of movements, like getting out of a chair or leaning down to pick something up, were enough for the implant to fracture inside patients’ bodies.
A broken artificial hip requires lengthy and often traumatic revisionary surgery. To repair it, orthopedic surgeons must crack open the patient’s femur to remove the faulty implant embedded in the thigh bone.
These surgeries have long, painful recoveries and often result in permanent disability, leaving the patient less able to participate in daily life than before their total hip replacement. Some patients who received a double hip replacement with the Profemur necks suffered fractures in both legs, often just a few years apart.
Yale University’s Dr. Lee Eric Rubin, an orthopedic surgeon and expert on prosthetic hips, told CBS News that tiny cracks formed on the neck over time, leaving patients with no idea there was a problem until it snapped.
“From a patient’s perspective, they’re walking around on what otherwise would seem like a successful hip implant,” he said. “And all of a sudden, as they took a step, they could not bear weight … like a black hole had developed under their foot.”
The necks, flexible 2-inch components linking the thigh and torso, allowed surgeons to customize the Profemur hip stem to the patient’s leg length, ostensibly for a more comfortable fit. They were intended to be less invasive than traditional total hip replacements and help preserve more tissue.
Profemur’s original manufacturer, medical device company Wright Medical, changed the neck’s metal from titanium to a stronger cobalt-chromium alloy in 2009. When those necks also began to break, Wright recalled one size but left 11 others on the market despite reports of another serious problem.
The cobalt-chromium necks, while less likely to fracture, were found to corrode against the stem’s titanium socket, leaking metallic ions into the patient’s bloodstream. Plaintiffs allege that this led to intense pain and swelling, a “debilitating” lack of mobility, nerve damage, and a host of neurological issues.
A number of scientific studies have concluded that the combination of the neck’s weak design and potential for corrosion significantly increased the chance of device failure.
The Profemur necks were approved for sale in 2000 by the FDA’s 510(k) program, which approves medical devices without thorough product testing if they are very similar to other approved devices. Wright Medical claimed the Profemur was equivalent to five other artificial hip devices, but those had differently designed necks; the FDA said the differences weren’t likely to be problematic.
Despite this initial confidence, current manufacturer MicroPort initiated nine FDA recalls of Profemur hip components over 13 years. The most recent in 2020 was a Class 1 recall, meaning that the modular neck has a “reasonable probability” of causing serious health problems or death.
The 2020 recall was the first to be permanent, despite FDA data showing that patients have reported fractures in the components since at least 2005.
The KFF Health/CBS News investigation found that many patients’ fractures could have been avoided had Wright Medical or the FDA responded to early reports with more urgency.
To date, at least 28 sizes of Profemur dual modular neck models have been reported fractured or corroding; just 11 of those have been permanently recalled. Microport’s website states that the dual modular neck models are no longer available in the U.S.
While an attorney for Wright Medical said in court that the company knew of 768 fractures out of about 353,000 Profemur necks sold – a rate of 0.2% - the 2020 recall showed a much higher fracture rate of 2.2%.
Artificial hips are intended to last 20 years or more with no complications, and more than 450,000 total hip replacement surgeries are successfully performed in the U.S. every year.
Wright Medical, which has made implantable medical devices since at least the 1970s, sold its hip and knee implant division to Chinese company MicroPort in 2013, including the Profemur. A large U.S. medical device company, Stryker Corp., acquired the remainder of Wright in 2020.
Plaintiffs in Profemur artificial hip lawsuits have alleged that MicroPort and Wright Medical knew or should have known of the risks involved in the dual modular necks and failed to warn physicians and patients of these risks.
The manufacturers, which have never admitted fault, have paid out at least $330 million in settlements since the first lawsuit was filed in 2015.
While most of the 1,800+ settled lawsuits were part of multidistrict litigation (MDL) that closed to new plaintiffs in 2018, the remaining unresolved cases were reconsolidated into MDL under the Eastern District of Arkansas in 2020. Any patient who suffered injury from a Profemur artificial hip might still qualify for a lawsuit and should contact an experienced product liability attorney.