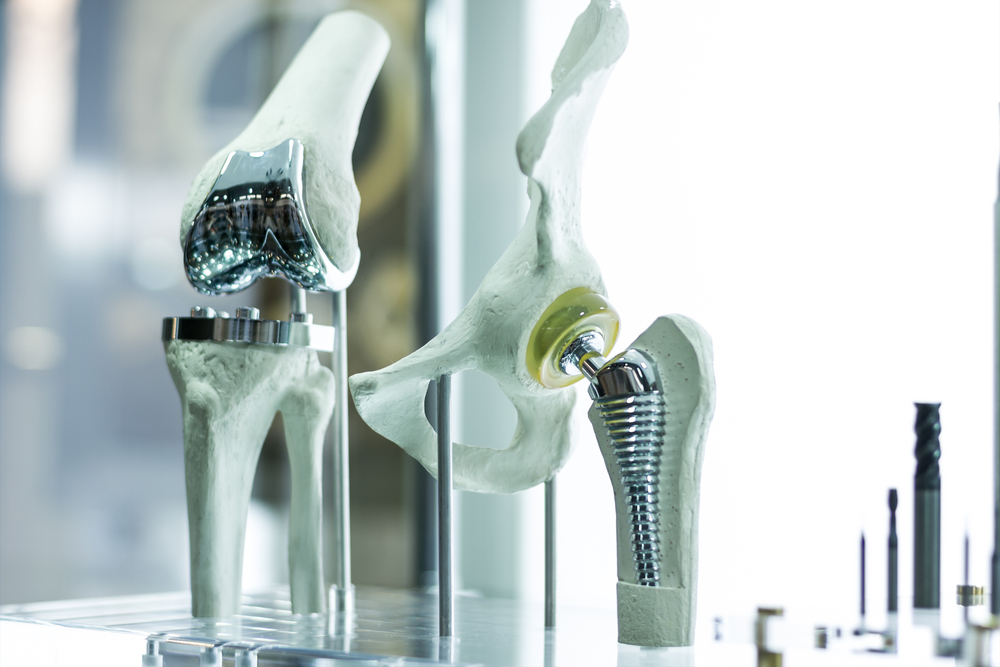
Medical device company Exactech has recalled hundreds of thousands of joint implants due to concerns that their polyethylene liners could degrade early and cause health complications. The first Exactech recall occurred in August 2021 and only included certain implants, but the manufacturer later expanded and recalled all ankle and knee implants produced after 2004.
Joint replacement surgery is one of the most common procedures in the U.S., with more than a million surgeries performed yearly. During the surgery, an orthopedic surgeon removes damaged cartilage and bone and replaces them with a prosthetic piece made of metal, plastic, or ceramic. For people with arthritis and joint injuries, the procedure reduces pain and dramatically improves their quality of life. When a joint implant is recalled, these patients wonder whether they’ll be negatively affected.
The success rate for joint replacement surgery varies. Patients often need revision surgery within 10 to 20 years of the procedure, and the follow-up procedure is more complicated and has more risks than the original surgery. The manufacturer found that the Exactech joint replacement parts degraded quickly because of a packaging issue. The device’s polyethylene liners were exposed to too much oxygen, making revision surgery necessary much earlier than expected.
Decreased joint function, pain, and swelling are telltale signs of joint implant failure. Not every Exactech recalled implant requires a second joint replacement surgery, but if it does, it can take several hours and require more specialized care. It has complications and risks like pulmonary embolisms, nerve damage, infection, and ossification.
Companies must notify consumers about medical implant device recalls, but customers might miss those advisories for various reasons — a change in address, a delay in a notification from the manufacturer, or not understanding that the recall might include their product or device. If you have an Exactech implant and haven’t had any complications, doctors don’t recommend getting it removed preemptively. But it’s still important to know whether you’re potentially affected by the recall, especially if you develop health problems. If you’re wondering whether your Exactech hip, knee, or ankle replacement implant is included in the recall, there are a few ways to find out.
Visit The Exactech Recall Website
First, you should gather details about your joint implant. Your medical records will include the precise implant you received along with the device’s serial number. You can then cross-reference this information on Exactech’s website, which has a comprehensive list of all the implants included in the recall. The searchable database consists of the product line, specific brand name, and the number of units affected. The company also has a telephone hotline for patients with questions about the recall. You’ll have the option to file a claim with Exactech for any out-of-pocket expenses caused by implant damage. However, it’s important to know that filing an Exactech lawsuit is a viable option and can lead to more compensation than settling with the company upfront.
Talk To Your Orthopedic Surgeon About Your Exactech Implant
The surgeon who operated will be able to answer questions about your Exactech implant and whether you should be concerned. Doctors must note an implant’s manufacturer, lot number, and serial number before using it during surgery. Even if your physician is no longer practicing or you’re no longer a patient of theirs, your medical records should be on file. If you do find out you have a recalled Exactech implant, telling your orthopedic surgeon is necessary. They’ll help you determine what warning signs you should look out for that may indicate joint implant failure.
Consult the FDA Database for Exatcech Implant Recalls
The FDA monitors the situation when a product is discovered to be defective. A company has the option to recall a product voluntarily, as Exactech did. If the manufacturer refuses, the FDA can force a recall. The administration has a Medical Device Recalls Database that includes detailed information about Exactech products, including serial numbers and the reasons given by the company for each recall. The government acts as an unbiased third-party source for consumers who want information about product recalls.
If you have an Exactech implant and are affected by product recalls, it’s essential to talk to an attorney before accepting any settlement offer from the company. Joint implant failure is a serious issue that can affect your earning potential and overall quality of life and cause needless pain and suffering. A lawyer will be able to help you explore your legal options and determine the best route to get the compensation you need.