Were Patients Notified of the Paragard IUD Label Change?
The Paragard IUD is well-known as a long-term form of birth control. It’s the only non-hormonal IUD out of the five IUDs available in the U.S., meaning its users won’t experience hormonal-based side effects like headaches or nausea.
However, it’s faced plenty of scrutiny over the years. Paragard has potential side effects, but much of its controversy surrounds its tendency to break during usage or removal.

Unfortunately, it turns out the manufacturer changed the label without warning Paragard users about these breakage concerns.
Below, we’ll discuss the issue of Paragard breakages and the events surrounding the manufacturer’s failure to warn about them in more detail.
Paragard IUD Breakage
As mentioned earlier, there have been many cases of Paragard IUDs breaking while a woman has it or during the removal procedure — especially the latter.
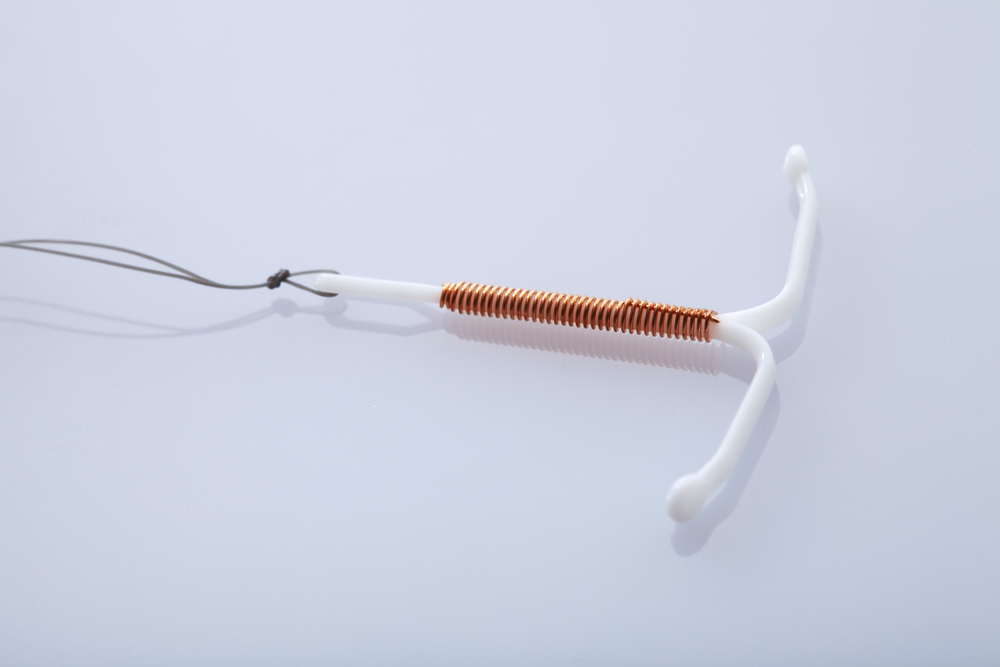
The device is designed to make removal easy. The arms are supposed to bend, and it has a thread attached to assist the medical professional in removing it. Yet, the arms fail to bend in many cases as the doctor attempts to remove the device. As a result, only the central portion is removed — the arms break off and are left behind in the uterus. This can lead to several potentially life-threatening injuries.
The FDA has previously warned partial or total perforation of the uterine wall or cervix may occur when the device is placed on rare occasions — although it may not be detected until later — while some have reported spontaneous migration.
If perforation happens, the FDA recommends prompt removal because the patient risks experiencing copper-related intraperitoneal adhesions. Additionally, devices left in the peritoneal cavity can penetrate or obstruct the intestines and/or damage adjacent organs.
These are reported in the FDA’s adverse event reporting system. Around 3,290 of these breakages were reported at the end of July, with about 2,000 considered “serious.” In fact, breakage is ranked as the fifth most common adverse event Paragard users experience.
The Paragard IUD Label Change
Around 2013, Paragard’s manufacturer — Connecticut-based CooperSurgical — began noticing and reporting an increased number of breakage-related complaints from users. As these reports added up, CooperSurgical decided they needed to update the device’s label, and they did so in 2019.
The exact change was worded as follows:
“Breakage of an embedded Paragard during non-surgical removal has been reported.“
This was added in the “Warnings and Precautions” section of the Paragard label, under the subsection labeled “Embedment,” which describes the risk of the device partially penetrating or embedding itself in the myometrium. The FDA has previously noted this may require surgical removal — a much longer process than the few-minute-long outpatient procedure of typical Paragard removal.
So the Paragard label was changed to mention the breakage risk. However, many women using the device were never notified of this label change, and it was buried down in the label. It turns out that the manufacturer was not required to notify women using the device of the change.
That said, patients are supposed to receive a card with Paragard information, but an investigation from Spotlight on America found that women using the device received differing information. Some received cards with their removal, others received a pamphlet with general information, and some didn’t get anything.
It’s also worth noting that when women go to get a Paragard device, they don’t necessarily see the warning label either since they don’t get the package the device comes in — unlike if they were to purchase something at a store. The doctor removes it from the box, so the patient won’t get a chance to see the warning label.
As a result, many women have been unaware of the breakage risk of Paragard. A broken Paragard device may be grounds to file a Paragard IUD lawsuit. If you or someone you know has used Paragard and experienced a breakage, you may want to contact an attorney for legal advice. You may be able to seek financial compensation for Paragard-related injuries.